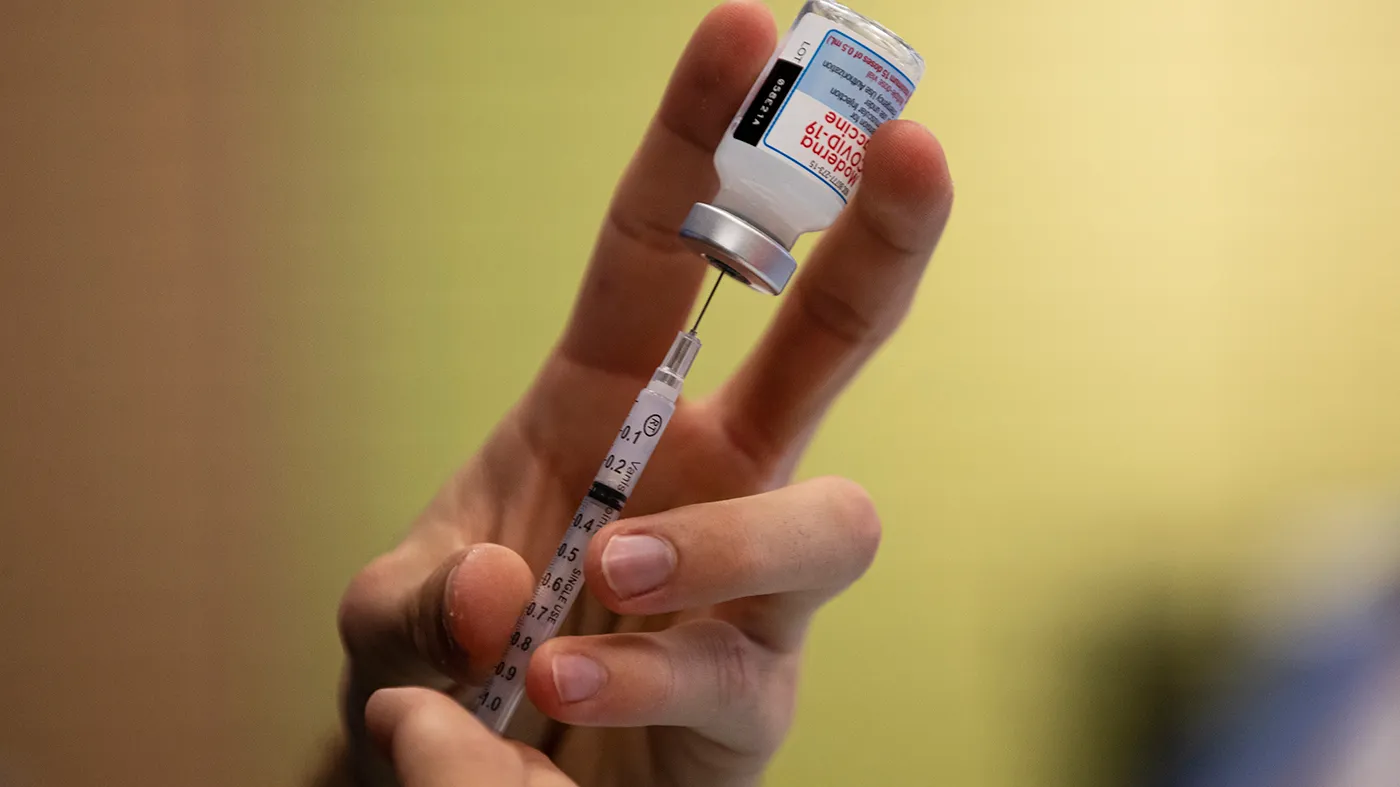
Health and Human Services (HHS) Secretary Robert F. Kennedy Jr.’s decision this week to cancel hundreds of millions of dollars in mRNA vaccine funding will leave the United States unprepared for the next pandemic and other public health emergencies, public health experts warned.
“I’ve tried to be objective & non-alarmist in response to current HHS actions—but quite frankly this move is going to cost lives,” President Trump’s former surgeon general, Jerome Adams, said in a post on the social platform X.
The first COVID-19 mRNA vaccines from Pfizer and Moderna hit the market in 2021, just a year after the virus first appeared. Vaccines typically take years to develop, but the mRNA shots were developed in record time thanks to a massive influx of funding from the first Trump administration, dubbed Operation Warp Speed.
The COVID shots proved safe and effective, and they helped bring about an end to the pandemic. Experts say mRNA technology has the potential to revolutionize treatments for evolving pathogens, especially bird flu, because the platform can be easily modified.
On Tuesday, Kennedy canceled $500 million worth of contracts related to mRNA vaccine research funded by the Biomedical Advanced Research and Development Authority (BARDA).
Instead, he said the agency will focus on platforms with “stronger safety records.”
Public health experts point out that reviews of the hundreds of millions, if not billions, of doses of mRNA shots administered worldwide have found very few adverse events.
Coller said the move will put the U.S. behind other nations in biomedical research and sends a “clear message” to scientists and the industry that it is not wise to invest in mRNA technology because if they do, they will likely not receive funding or approval from the federal government.
Jennifer Nuzzo, a professor of epidemiology and director of the Pandemic Center at the Brown University School of Public Health, told The Hill that the cancellation is a threat to national security, as it opens the U.S. to future public health emergencies caused by biological warfare.
“One of the ways that we deter that from happening is to say the United States is absolutely committed to preparedness,” Nuzzo said. “When we take them off the table and leave nothing in their place, we basically signal to our adversaries that we are no longer interested in defending ourselves.”
In the long run, Nuzzo said, winding down research efforts on mRNA vaccine platforms will stifle medical innovation coming out of the U.S., including new treatments for diseases like cancer.
“It’s troubling on a number of fronts,” she said.
That same general approach could be used to help potentially vaccinate someone against a particular cancer, according to Nuzzo.
“Cancers have a genetic signature,” she said. “We don’t know what’s going to happen but there are some preliminary studies that suggest it could be a very promising way to treat cancers.”
“I think you’re going to see anyone who is willing to invest in mRNA technology, pulling back, if not completely getting out of this business,” he said.
“This is terribly unfortunate, given that we have a number of other infectious diseases for which this vaccine platform was being explored in terms of taking on other infectious diseases and including some cancer vaccines.”
In 2021, he falsely said the mRNA COVID-19 vaccine was the “deadliest vaccine ever made.” Kennedy has also been facing pressure from fellow antivaccine activists for not doing enough to keep mRNA vaccines off the market.
He was criticized earlier this year when the Food and Drug Administration (FDA) approved Moderna’s updated COVID-19 vaccine, even though the agency limited its use in children.
“One mutation and the vaccine becomes ineffective,” Kennedy said.
Following the announcement, public health experts urged Congress to restore funding for mRNA vaccine research, with some calling it an “assault” on federal vaccine policy.
“The mRNA vaccine platform was essential to rapid vaccine development and deployment during the COVIVD-19 pandemic and remains essential to preparing for future public health emergencies.”
But the full extent of Kennedy’s move isn’t known.
Gritstone bio, another company on the HHS list of canceled contracts, ceased operations earlier this year after the company filed for bankruptcy in 2024.
A spokesperson for Tiba Biotech said HHS’s decision to terminate their BARDA contract “comes as a surprise” since its contract was for a therapeutic, not a vaccine, and it does not use mRNA technology. Instead, it uses a method called RNA interference, which the FDA approved to treat some diseases in 2018.